Chemistry of fluorinated phosphonates
We have developed new methodologies for nucleophilic transfer of fluorine-containing groups using fluorinated phosphonates (Chemické Listy 108: 926, 2014).
- Trifluoromethylation
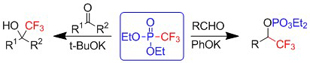
Diethyl trifluoromethylphosphonate affects trifluoromethylation of non-enolizable ketones to trifluoromethyl-containing alcohols. It also reacts with aldehydes to give phosphates of trifluormethylated alcohols (Tetrahedron Letters 51: 252–255, 2010).
- Difluoromethylation
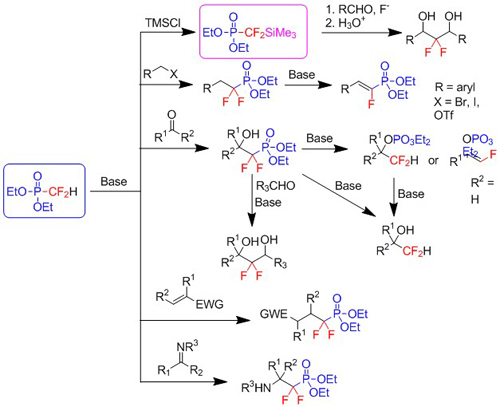
Diethyl difluoromethylphosphonate represents an entry to a range of difluoromethyl, difluoromethylene and fluorovinyl groups containing compounds (Tetrahedron 64: 10977-10985, 2008; Synthesis 957-962, 2009; Journal of Fluorine Chemistry 130: 493-500, 2009; Synlett 331-334, 2011; Journal of Fluorine Chemistry 137: 34-43, 2012; Journal of Fluorine Chemistry 141: 76-82, 2012.
- Fluoromethylation
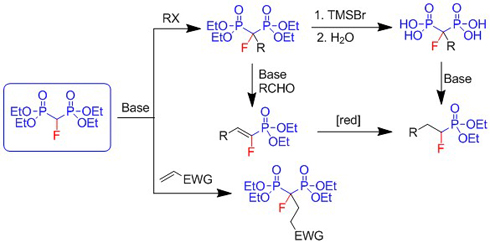
Tetraethyl fluoromethylenebisphosphonate serves as precursor to α-alkyl-α-fluoromethylenebisphophonates, α-alkyl-α-fluoromethylenebisphophonic acids, α-fluorovinylphosphonates and α-fluorophosphonates (Organic & Biomolecular Chemistry 9: 4035-4038, 2011; Synlett 331-334, 2011; Journal of Fluorine Chemistry 132: 363-366, 2011.
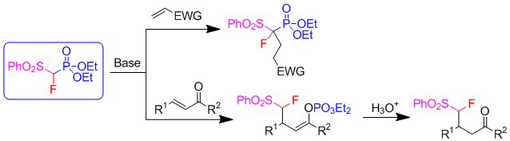
- The reactivity of McCarthy's reagent with enones and other α,β-unsaturated compounds was investigated (Journal of Organic Chemistry 78: 4573-4579, 2013).
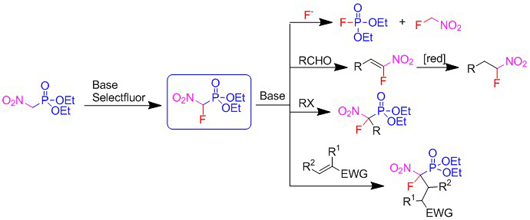
- Diethyl fluoronitromethylphosphonate is a new reagent for Horner reaction with aldehydes and nucleophilic fluoroalkylation (Chemistry - A European Journal 20: 1453-1458, 2014).