New methodologies for the preparation of (pentafluorosulfanyl)benzenes
Organic compounds with pentafluorosulfanyl (SF5) groups display a unique set of physicochemical properties. This includes extreme kinetic and hydrolytic stability, very strong electron acceptor capability, and high lipophilicity with high SF5 electronegativity. A very high dipole moment can be achieved by the introduction of SF5 group without increasing molecular polarity. These properties make the pentafluorosulfanyl group an increasingly interesting structural motif for the design of bioactive compounds, including agrochemicals and pharmaceuticals as well as functional materials such as polymers or liquid crystals. However, access to SF5-containing compounds is very limited and their chemistry remains largely unexplored.
In this project we are developing new methodologies towards substituted (pentafluorosulfanyl)benzenes (Organic Letters 13: 1466-1469, 2011; Journal of Organic Chemistry 76: 4781-4786, 2011; Tetrahedron Letters 52: 4392-4394, 2011; Journal of Fluorine Chemistry 143: 130-134, 2012; European Journal of Organic Synthesis 2123-2126, 2012; Beilstein Journal of Organic Chemistry 1185-1190, 2012; Beilstein Journal of Organic Chemistry 9: 411-416, 2013; Synlett 855-859, 2013; Journal of Organic Chemistry 79: 8906-8911, 2014; Environmental Science and Pollution Research 21: 753-758, 2014; Journal of Fluorine Chemistry 157: 79-83, 2014; Beilstein Journal of Organic Chemistry 11: 1494-1502, 2015; Chemical Communications 52: 7237-7240, 2016; Beilstein Journal of Organic Chemistry 12: 192–197, 2016; Beilstein Journal of Organic Chemistry 12: 110–116, 2016).
In this project we are developing new methodologies towards substituted (pentafluorosulfanyl)benzenes (Organic Letters 13: 1466-1469, 2011; Journal of Organic Chemistry 76: 4781-4786, 2011; Tetrahedron Letters 52: 4392-4394, 2011; Journal of Fluorine Chemistry 143: 130-134, 2012; European Journal of Organic Synthesis 2123-2126, 2012; Beilstein Journal of Organic Chemistry 1185-1190, 2012; Beilstein Journal of Organic Chemistry 9: 411-416, 2013; Synlett 855-859, 2013; Journal of Organic Chemistry 79: 8906-8911, 2014; Environmental Science and Pollution Research 21: 753-758, 2014; Journal of Fluorine Chemistry 157: 79-83, 2014; Beilstein Journal of Organic Chemistry 11: 1494-1502, 2015; Chemical Communications 52: 7237-7240, 2016; Beilstein Journal of Organic Chemistry 12: 192–197, 2016; Beilstein Journal of Organic Chemistry 12: 110–116, 2016).
Structure of selected aromatic SF5-compounds was investigated in collaboration with Prof. Slawin from St. Andrews University, UK (Structural Chemistry 28: 723, 2017). Bacterial degradation of some aromatic SF5-compounds was studied in collaboration with Prof. Murphy from Dublin, Ireland (Biodegradation 29: 259, 2018).
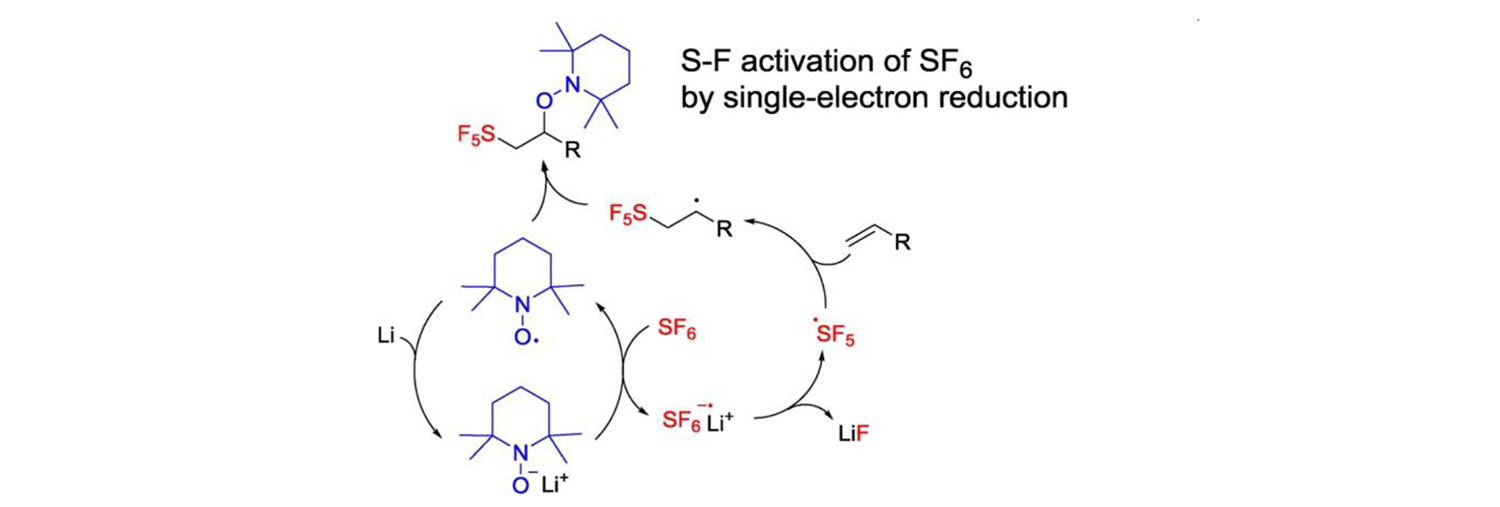
S-F activation of sulfur hexafluoride for the preparation of SF5-compounds is a very challenging project. We contributed to the progress of the field by studying reductive activation of SF6 with TEMPOLi (Journal of Fluorine Chemistry 213: 51, 2018).
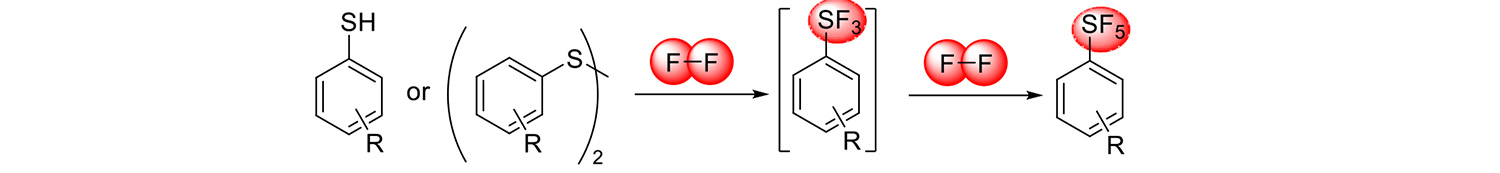
Availability of aromatic SF5-compounds is very limited. Our detailed study of the scope and mechanism of direct fluorination of aromatic thiols and disulfides with fluorine gas provided access to new SF5-benzenes (Chemistry – A European Journal 25: 11375, 2019).
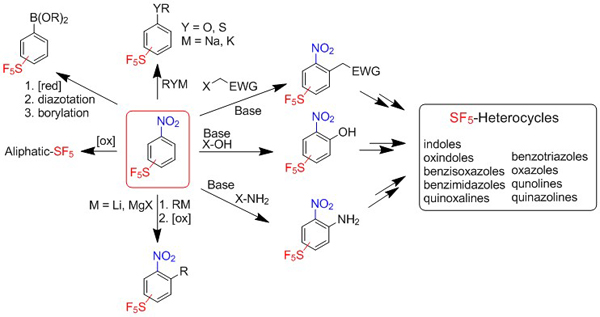
Methods available for the preparation of aromatic sulfur pentafluorides was reviewed in a recent book (Emerging Fluorinated Motifs: Synthesis, Properties, and Applications, Eds. J.-A. Ma, D. Cahard, 551-570, Wiley-VCH, 2020).


