Tetrafluoroethyl and tetrafluoroethylene group transfer
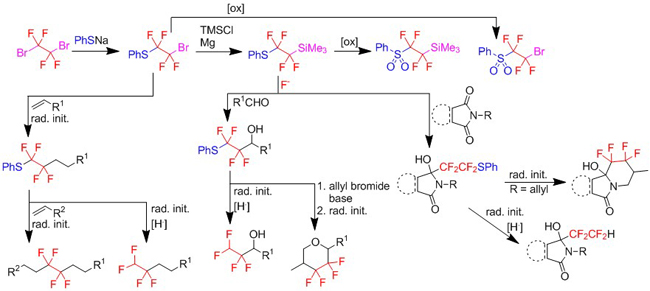
Starting from dibromotetrafluoroethane we have synthesized a variety of nucleophilic and radical sulfur-containing compounds, which are now under investigation as reagents for tetrafluoroethyl and tetrafluoroethylene group transfer (European Journal of Organic Chemistry 4528-4531, 2011; Synlett 1187-1190, 2012; Journal of Fluorine Chemistry 156: 307-313, 2013; Journal of Fluorine Chemistry 171: 162-168, 2015; Chemistry - A European Journal 417-424, 2016).
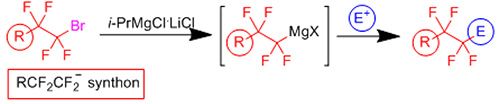
Magnesiation of tetrafluoroethyl-containing bromides with Turbo Grignard reagents led to the formation of organomagnesium compounds, which were stable at low temperature and reacted with various electrophiles to afford novel functionalized tetrafluoroethylene-containing products (Organic Letters 18: 5844-5847, 2016).
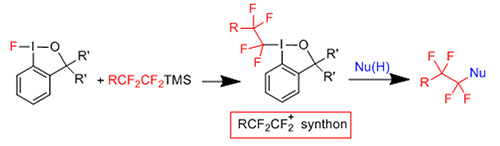
In collaboration with the group of Prof. Togni (ETH, Zurich) a series of new hypervalent iodine reagents was prepared and used for electrophilic CF2CF2 transfer (Chemistry - A European Journal 22: 417-424, 2016).
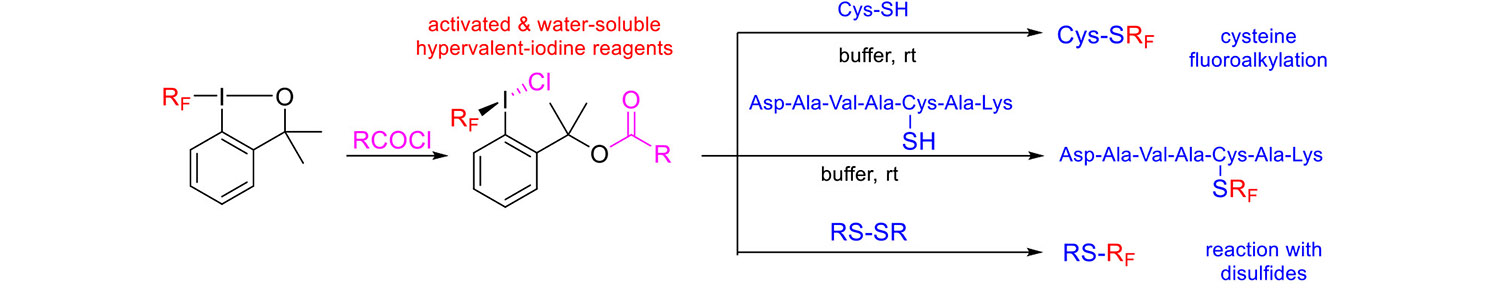
The exceptionally good reactivity and selectivity of these hypervalent iodine reagents toward thiols prompted us to investigate conjugation to biological thiols. A secondary amine platform reagent was designed, converted to amide, sulfonamide or termiary amine and in collaboration with Prof. Hilvert (ETH Zurich) studied for bioconjugation of a retroaldolase enzyme (Chemistry - A European Journal 23: 6490-6494, 2017). Our reagents have several advantages over existing commercial thiol conjugation reagents: modular synthesis with a variety of functional groups attached in the last step (fluorophore, biotin, PEG, reactive handle), exceptional selectivity for thiols, fast reaction rates and high stability of the conjugate.
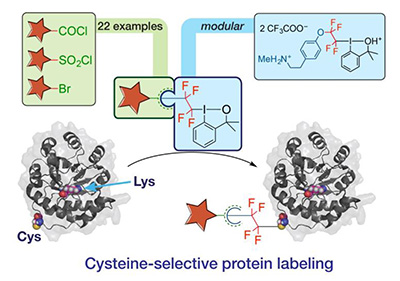
New water-soluble hypervalent iodine reagents for fluoroalkylation of biological thiols were prepared (Organic and Biomolecular Chemistry 17: 10097, 2019).
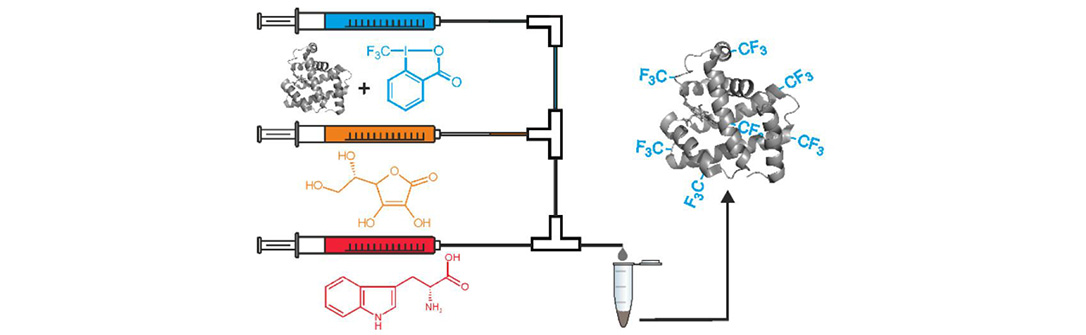
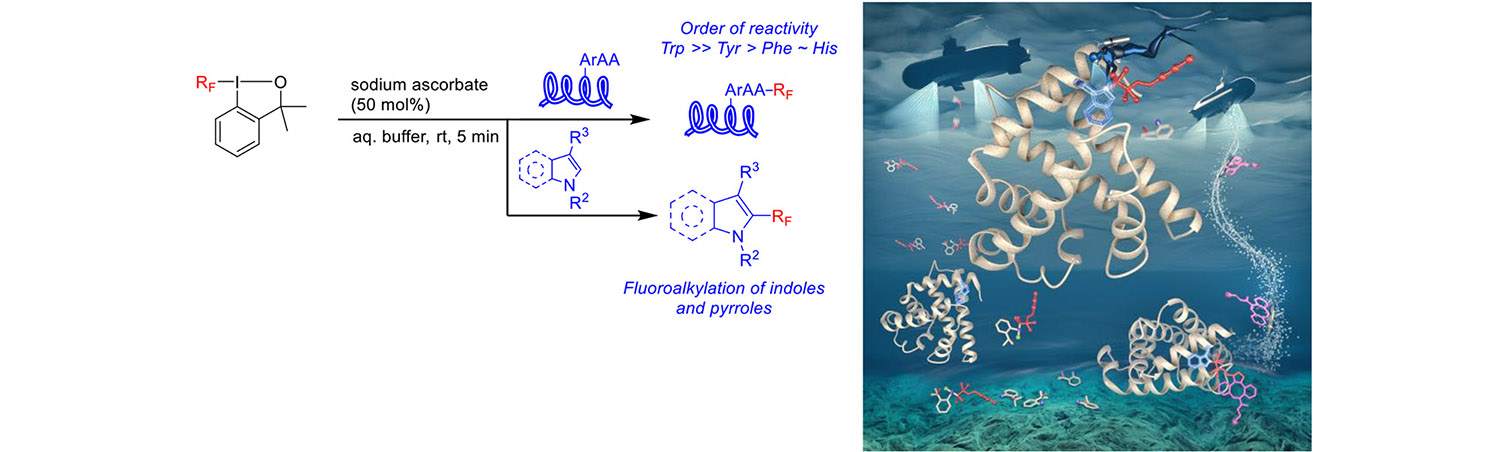
Cyclic hypervalent iodine reagents used for fluoroalkylation of thiols can be used also for fluoroalkylation of tryptophan and other aromatic amino acids. This was achieved by adding a suitable water-soluble and biocompatible reductant (sodium ascorbate) which induces the formation of fluoroalkyl radicals. The process was demonstrated on numerous indoles, peptides and proteins where targeting Trp was very selective (Chemistry – A European Journal 25: 15779).
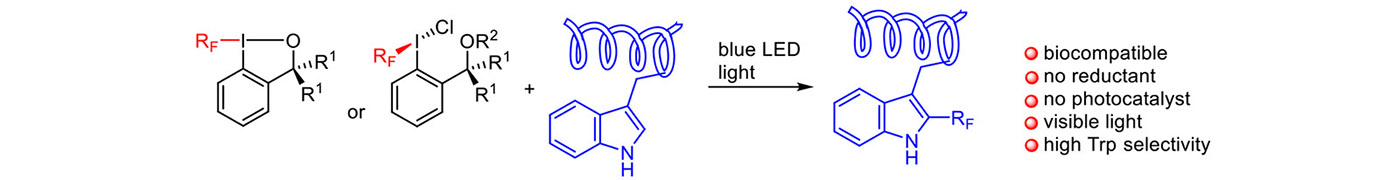
Interestingly, without the reductant in the presence of a blue light the iodine reagents are decomposed to form fluoroalkyl radicals as well. The method has been applied for derivatization of Trp-containing biomolecules (ChemPhotoChem: in press).
The chemistry of tetrafluoroethylene-containing compounds was recently reviewed (European Journal of Organic Chemistry: 3554, 2018).
We collaborated with Petr Novák from Charles University and BIOCEV on the development of fast fluoroalkylation of proteins using hypervalent iodine compounds and a biocompatible reductant. These modification uncovered the structure and dynamics of protein complexes and protein-protein interactions (Journal of the American Chemical Society 143: 20670-20679, 2021).