The chemistry of polyfluorinated azides
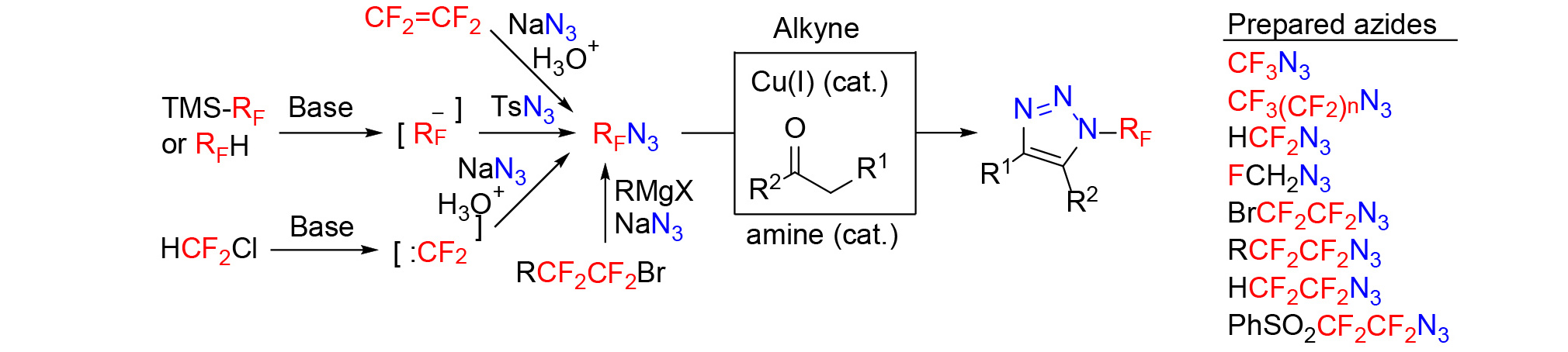
In 2017, we reported a breakthrough in azide chemistry. CF3N3 and longer carbon chain analogues were prepared from silanes or other carbanion precursors, which opened a door to study properties and reactivity of these compounds. They are no longer regarded as chemical curiosities. Furthermore, they were shown to be stable and not explosive (Angewandte Chemie International Edition 56: 346, 2017). Subsequently, other fluorinated azides such as HCF2N3 (European Journal of Organic Chemistry 5087, 2018), BrCF2N3, BrCF2CF2N3 (Journal of Organic Chemistry 85: 11482, 2020), RCF2CF2N3 (Organic and Biomolecular Chemistry 15: 4962, 2017) and FCH2N3 (Organic Chemistry Frontiers 7: 10, 2020), PhSO2CF2CF2N3 (Journal of Organic Chemistry 88: 6939, 2023), and HCF2CF2N3 (Journal of Organic Chemistry 88: 14969, 2023) were prepared. They readily underwent copper(I)-catalyzed azide-alkyne cycyloadditions (CuAAC) and enamine-mediated azide-ketone cycloadditions (ChemistrySelect 3: 7045, 208) to form novel N-perfluoroalkyl triazoles. Preparation and reactivity of fluorinated azides was recently reviewed (Chemistry – A European Journal 773, 2020, Aldrichimica Acta 55: 37, 2022).
In the presence of superacids, azidotrifluoromethane protonates on nitrogen one to trifluoromethylamino diazonium cations (CF3NHN2)+ (Angewandte Chemie International Eddition 59: 12520, 2020) (collaboration with the University of Southern California).
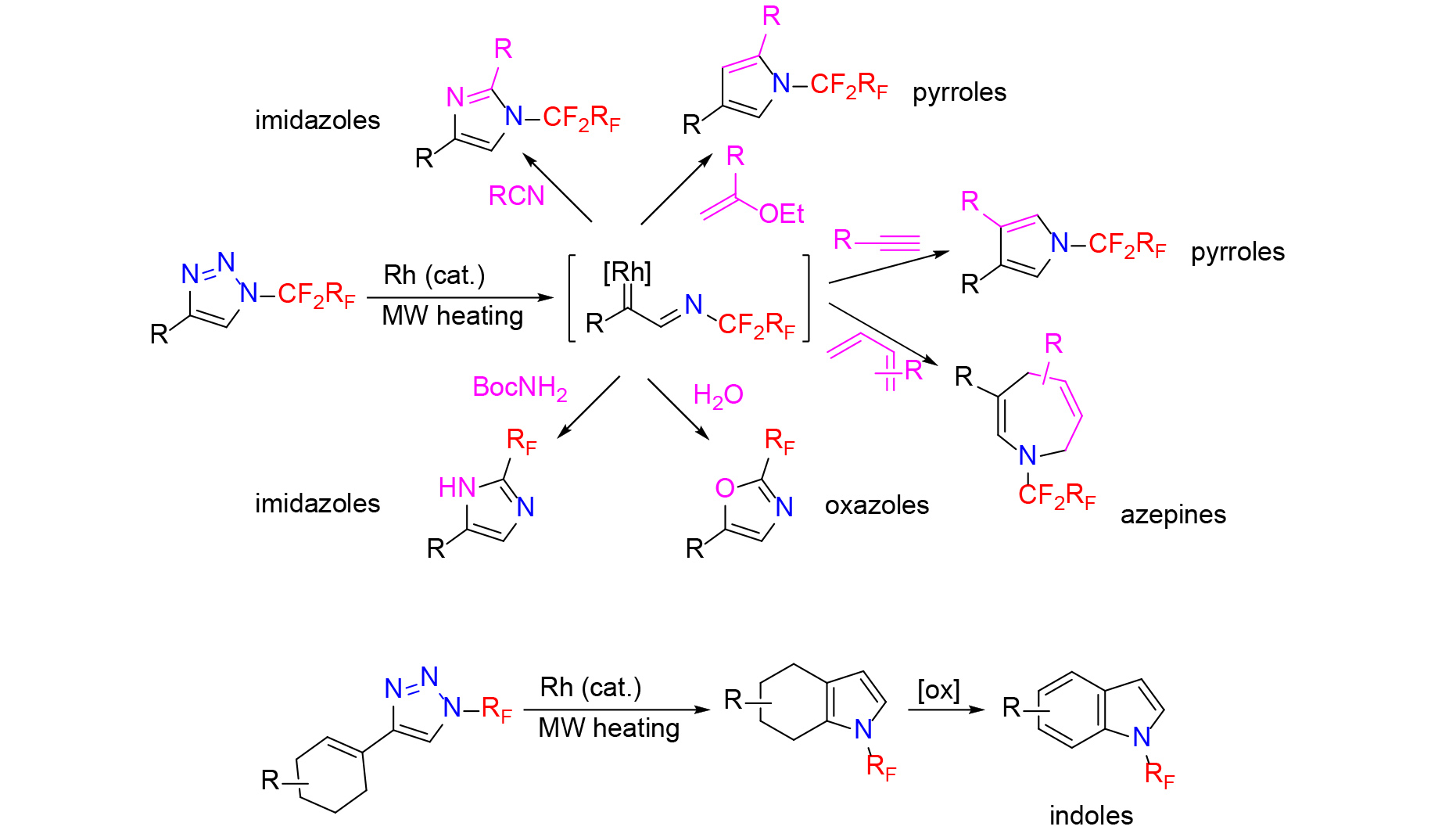
Heating N-(per)fluoroalkyl triazoles in the presence of rhodium catalyst allows to enter rhodium carbene chemistry for the synthesis of novel N-(per)fluoroalkyl pyrroles, imidazoles, azepines, oxazoles, thiazoles and other heterocycles (Chemical Communications 54: 3258, 2018, Journal of Organic Chemistry 83: 15195, 2018, Organic Chemistry Frontiers 6: 3776, 2019), Beilstein Journal of Organic Chemistry 17: 504, 2021). 4-Cyclohexenyl-substituted N-(per)fluoroalkyl 1,2,3-triazoles undergo transannulation to provide N-(per)fluoroalkyl indoles after oxidation (Organic & Biomolecular Chemistry 21: 7924, 2023).
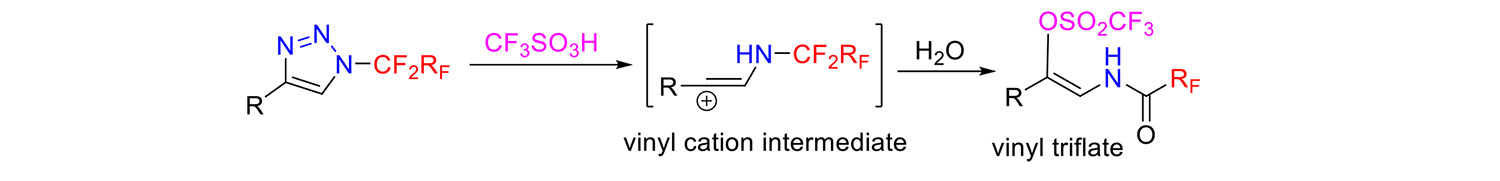
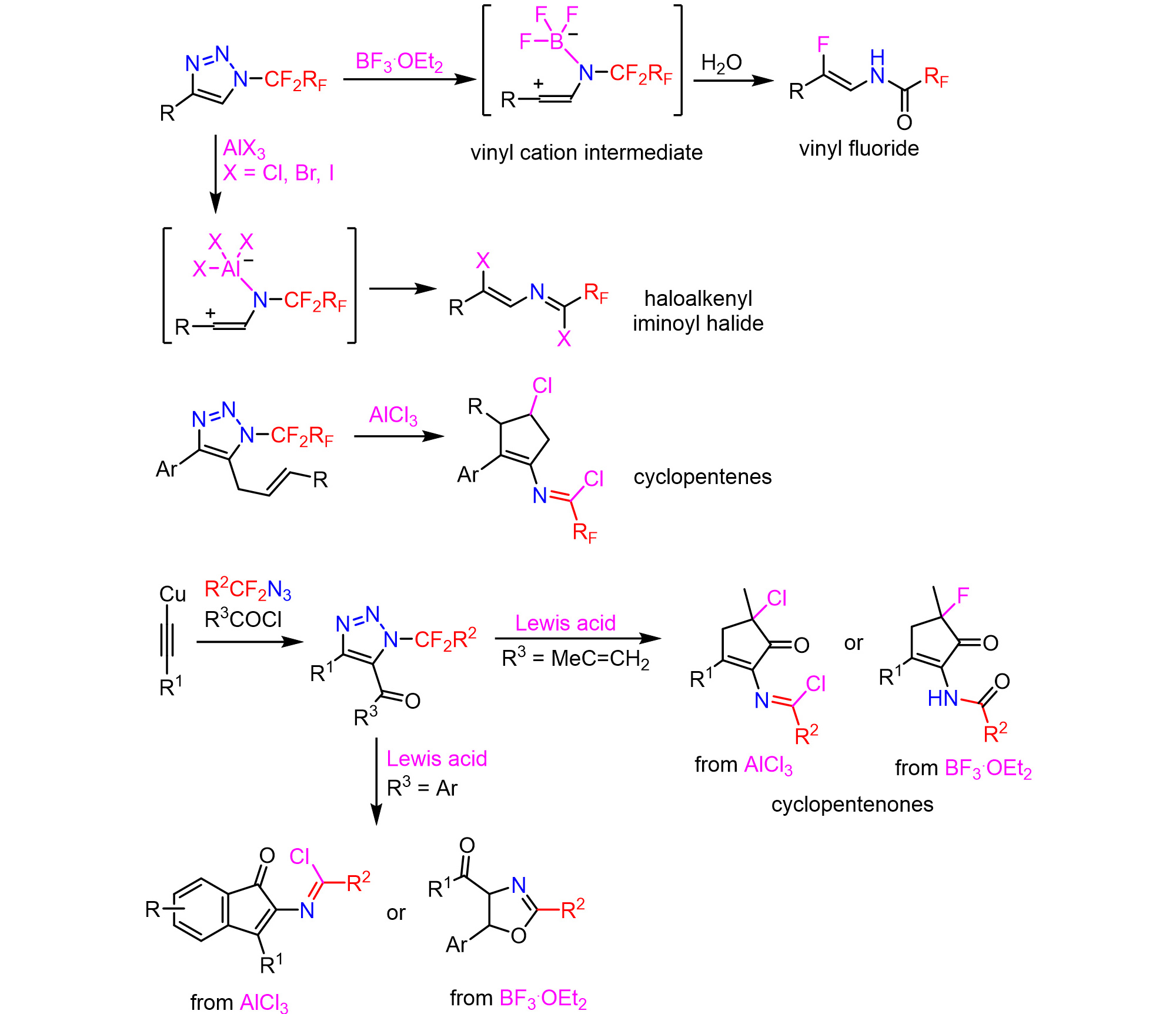
A new reactive mode was discovered in N-(per)fluoroalkyl triazoles. In the presence of superacids, such as triflic acid or fluorosulfonic acid, the triazoles ring open and vinyl triflates enamides are formed via a vinyl cation (Chemistry – A European Journal 25: 7640, 2019).
This reactive mode was extended to Lewis acids – in the presence of boron trifluoride β-enamido fluorides are formed (Organic Letters 23: 4224, 2021) and with aluminium halides highly functionalized haloalkenyl imidoyl halides are produced (Advanced Synthesis & Catalysis 363: 3528, 2021). The utilization of the vinyl cation intermediates for intramolecular C-C bond formation was also demonstrated (Journal of Organic Chemistry 88: 1155, 2023). Acylated triazoles were prepared by intercepted click reaction. The follow-up denitrogenation by the addition of Lewis acids led to cyclopentenones, oxazoles or indenones (RSC Advances 14: 13640, 2024).
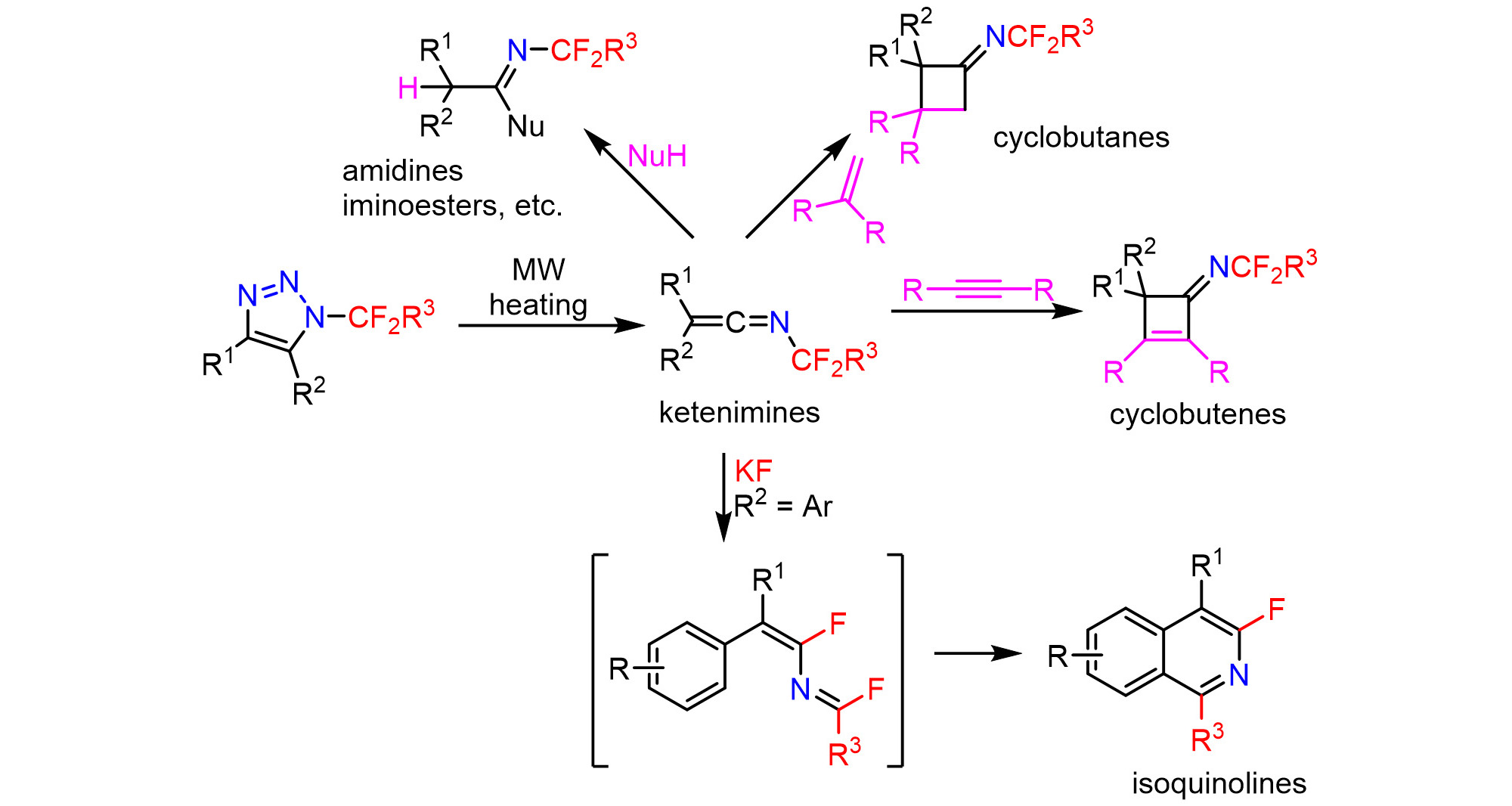
Microwave heating of N-(per)fluoroalkyl-1,2,3-triazoles led to denitrogenation and rearrangement to provide stable ketenimines, which underwent addition of nucleophiles or [2 + 2] cycloadditions (Organic Chemistry Frontiers 10: 3201, 2023). Potassium fluoride-mediated induced the 1,3-fluorine shift and led to the formation of isoquinolines (Organic Chemistry Frontiers 12, 4442, 2024).
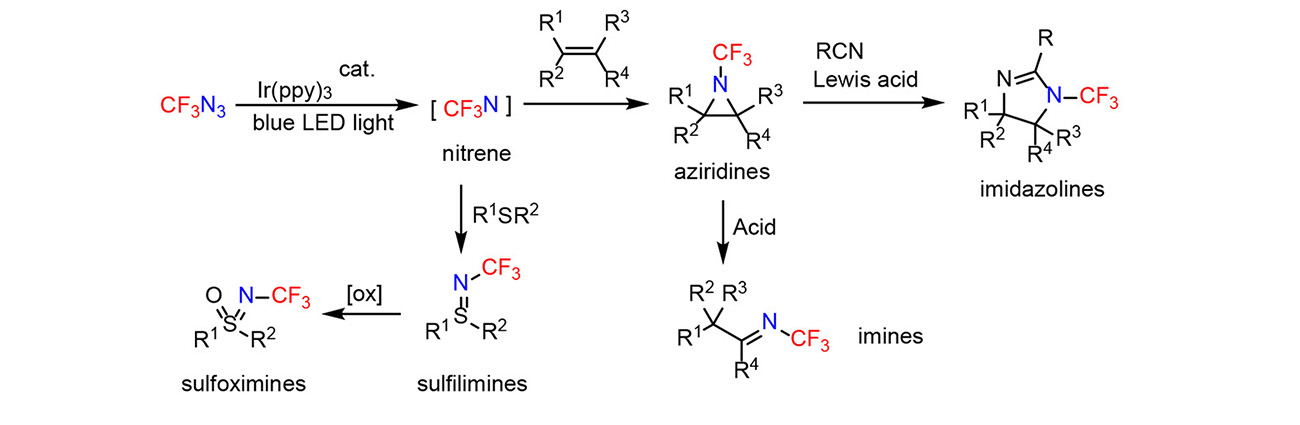
Triplet trifluoromethyl nitrene was generated photocatalytically from trifluoromethyl azide. The nitrene intermediate added to alkenes to form new aziridines, which underwent Lewis acid-mediated reactions with nitriles to imidazolines or acid-mediated rearrangement to N-CF3 imines (Angewandte Chemie International Edition 63: e202315162, 2024).
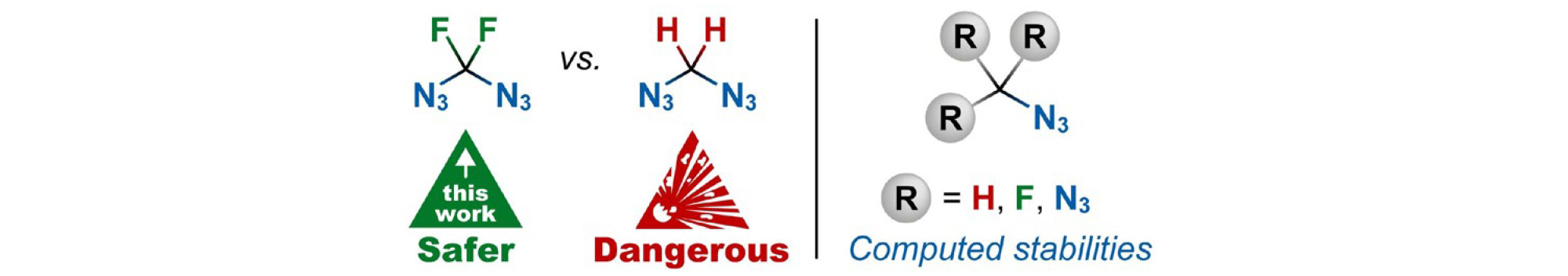
Diazidodifluoromethane, the first stable diazidomethane was prepared and stabilities of fluorinated and nonfluorinated organic azides was evaluated computationally (Chemical Communications 61: 885, 2025).
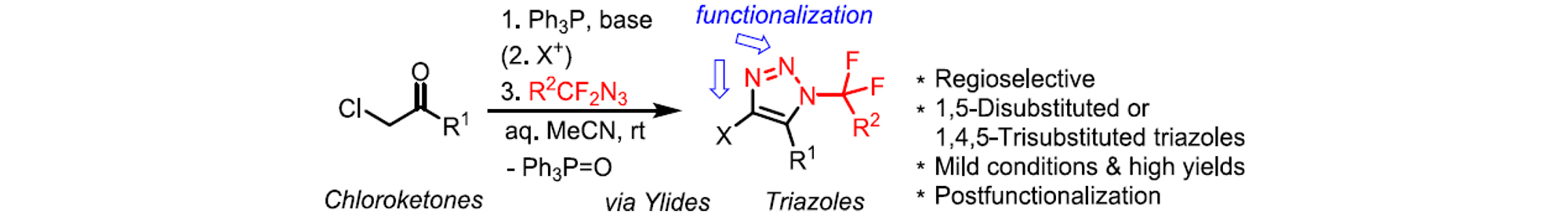
Cyclization of azidofluoroalkanes with carbonyl-stabilized phosphonium ylides resulted in the formation of 5-substituted triazoles (Journal of Organic Chemistry 90:11838, 2025).
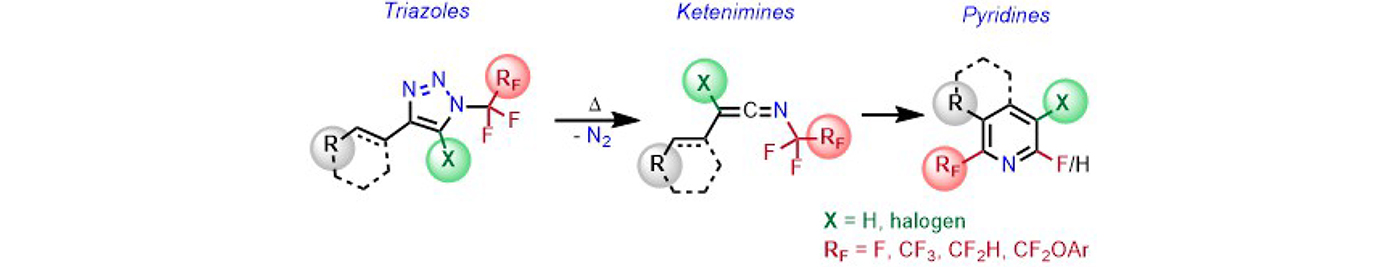
2-Fluoroalkylated pyridines and fluoropyridines were synthesized via ketenimines derived by thermal denitrogenation of triazoles (Journal of Organic Chemistry 91: 2232, 2026).